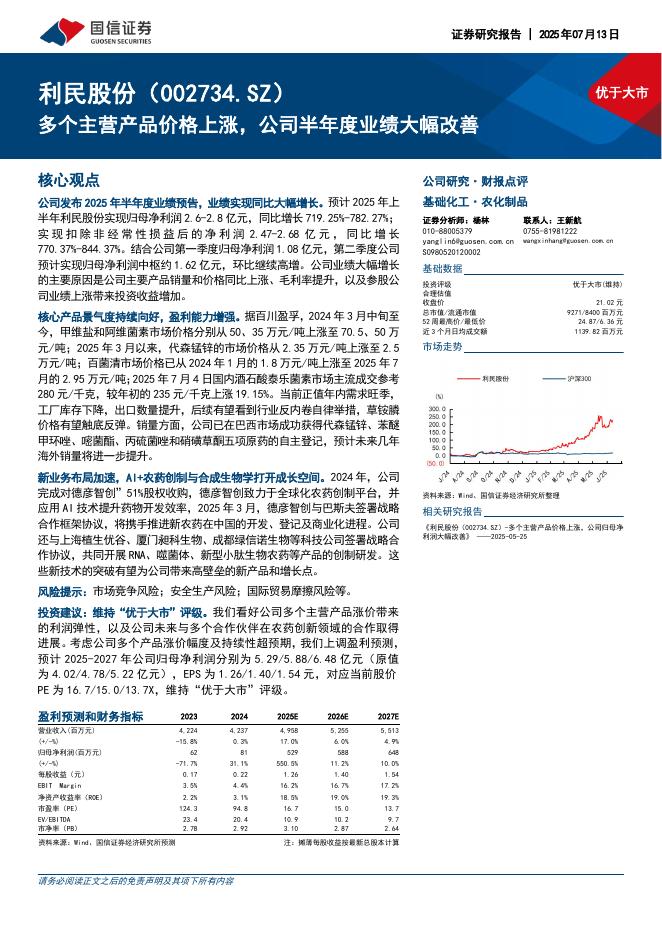
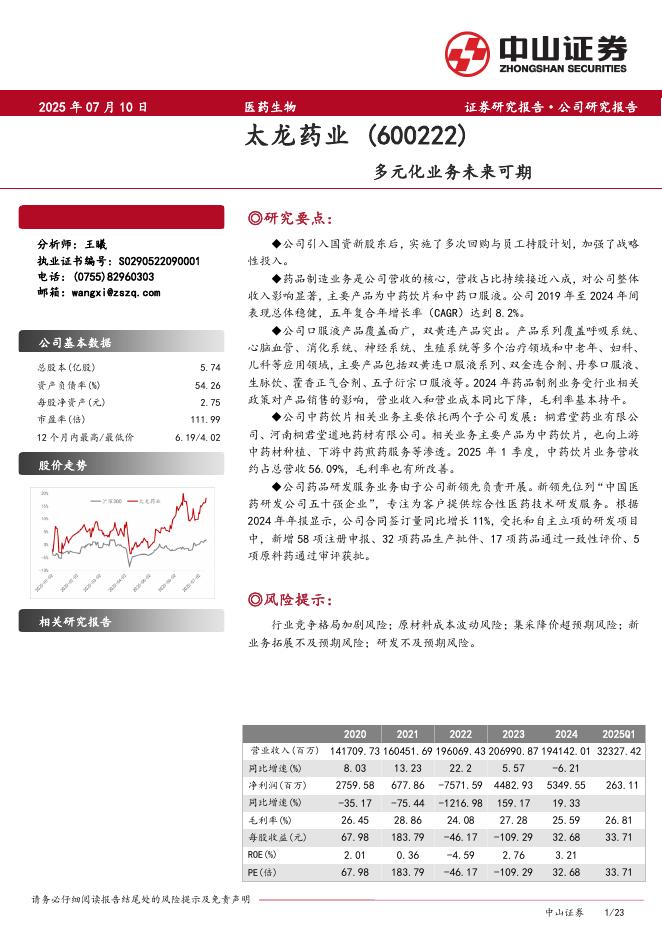
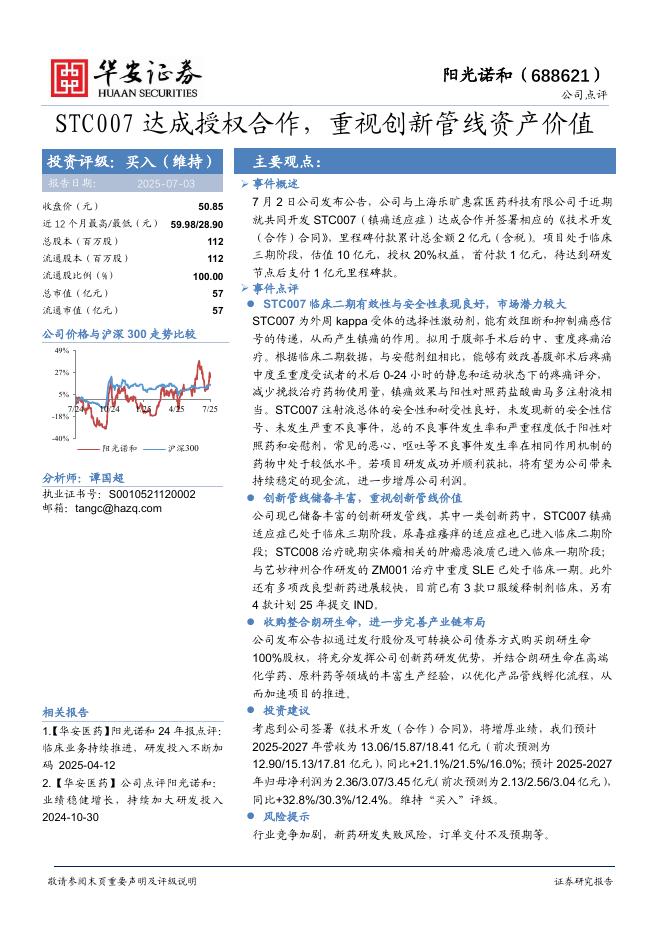
欧洲疾控中心:传染病威胁报告(2025年7月12日至18日,第29周)(英文版).pdf |
下载文档 |
资源简介
On 11 July 2025, EMA’s Pharmacovigilance Risk Assessment Committee (PRAC) concluded its review of the live-attenuated chikungunya vaccine Ixchiq. The temporary age restriction for adults aged 65 and older has been lifted because this is the age group in which chikungunya can be severe. However, PRAC strongly emphasises that Ixchiq should only be used when there is a significant risk of chikungunya infection and after a careful benefit-risk evaluation in all age groups. Seasonal surveillance o
已阅读到文档的结尾了